11/04/2022
Kirill Zhudenkov et al.
CPT Pharmacometrics Syst Pharmacol. 2022 Apr;11(4):425-437
•Clinical trials investigate treatment endpoints that usually include measurements of pharmacodynamic and efficacy biomarkers.
•Joint modeling is an advanced statistical methodology that allows for the investigation of clinical trial outcomes by quantifying the association between baseline and/or longitudinal biomarkers and event risk.
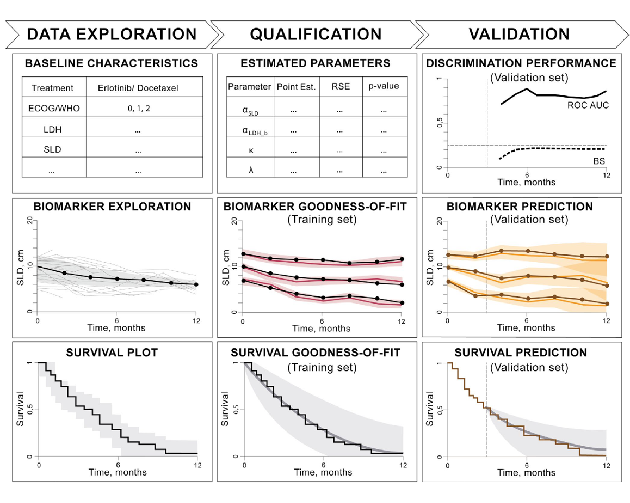
•Using an exemplar data set from non-small cell lung cancer studies, we propose and test a workflow for joint modeling.
•It allows a modeling scientist to comprehensively explore the data, build survival models, investigate goodness-of-fit, and subsequently perform outcome predictions using interim biomarker data from an ongoing study.
Please, see a full text article (CPT Pharmacom Syst Pharma – 2022 – Zhudenkov.pdf)